VHC Vitamin-D
Quantitative Vitamin D Test [REF] 1000J-10 / 1000J-25 / 1000J-50
NOTE: THE ORIGINAL/REFERENCE VERSION OF THIS DOCUMENT IS THE ENGLISH VERSION.
A Rapid “Sandwich” lmmunochromatographic Test for the Quantitative Detection
of total 25-0H Vitamin D in Human Whole Blood
For In Vitro Diagnostic use only – Read Instructions before use
INTENDED USE
The VHC Vitamin-D Quantitative Vitamin D Test is an immunochromatography-based one step in vitro test. It is designed for the quantitative determination of total 25-hydroxy Vitamin D (25-OH Vitamin D) in human finger-prick blood. This assay provides a preliminary diagnostic test result and can be used for screening of Vitamin D deficiency. The liquid chromatography with tandem mass spectrometry (LC-MS/MS) assays or other quantitative immunoassays are recommended to further confirm the diagnostic test results.
SUMMARY AND EXPLANATION
Vitamin D is a steroid hormone responsible for enhancing intestinal absorption of calcium and the regulation of its homeostasis. The two common forms of Vitamin D are Vitamin D2 and Vitamin D3. Vitamin D3 is naturally produced in the human skin through the exposure to ultraviolet light and Vitamin D2 is mainly obtained from foods. Vitamin D is transported to the liver where it is metabolized to 25-hydroxy Vitamin D. In medicine, a 25-hydroxy Vitamin D blood test is used to determine Vitamin D concentration in the body. The blood concentration of 25-hydroxy Vitamin D is considered the best indicator of Vitamin D status.
Vitamin D deficiency is now recognized as a global epidemic. Virtually every cell in our body has receptors for Vitamin D, meaning that they all require “Sufficient” Level of Vitamin D for adequate functioning. The health risks associated with Vitamin D deficiency are far more severe than previously thought. Vitamin D deficiency has been linked to various serious diseases: Osteoporosis, Osteomalacia, Multiple Sclerosis, Cardiovascular Diseases, Pregnancy Complications, Diabetes, Depression, Strokes, Autoimmune Diseases, Flu, Different Cancers, Infectious Diseases, Alzheimer, Obesity and Higher Mortality etc. Therefore, now detecting (25-OH) Vitamin D level is considered as “Medically Necessary Screening Test”, and maintaining sufficient levels not just to improve bone health, but to improve overall health and well-being.
Multiple guidelines for Vitamin D deficiency have been published by various health organizations; but a common recommendation remained to be established. Recent literature (Worm 2010) has suggested a level classification for the classification of Vitamin D status (see table underneath). According to recent literature a level underneath 20 ng/ml is to be considered as critical low and it is recommended to contact a therapist for further diagnostics and treatment. Vitamin D levels between 40 and 60 ng/ml are regarded as optimal and have shown preventive effects. The multiple preventive effects of optimized Vitamin D are reflected by the health claims approved of the European Union regarding Vitamin D supplements: supporting conservation of normal Calcium levels, normal bones, normal muscle function, normal teeth, and normal function of the immune system.
[table id=2 /]Note:
Vitamin D concentrations are reported either in [ng/ml] or [nmol/l] – (1 ng/ml is approximately 2.5 nmol/l). Make sure to check what unit system is used when comparing different test results!
TEST PRINCIPLE
VHC Vitamin-D Quantitative Vitamin D Test utilizes the principle of immunochromatography, a unique two-site “Sandwich” immunoassay on a membrane. The test employs a very “Exclusive” pair of anti-25-OH Vitamin D Monoclonal Antibodies; one conjugated with colloidal gold and another one immobilized on the solid phase. This will selectively detect Vitamin D with a high degree of sensitivity and specificity.
As the test sample flows through the membrane assembly within the test device, the colored anti-25-OH Vitamin D-colloidal gold conjugate complexes with 25-OH Vitamin D from the sample. This complex moves further on the membrane by the capillary action to the test region (T) where it is immobilized by another anti-25-OH Vitamin D coated on the membrane, leading to formation of a pink / purple colored band, which confirms a positive test result. The intensity of colored band in the test line region is 25-OH Vitamin D concentration-dependent, higher the concentration of 25-OH Vitamin D in the tested sample, the stronger the colored band is. A control line is present in the test window to work as procedural control. This colored band should always appear on the control line region (C) if the test device is stored in good condition and the test is performed appropriately.
MATERIALS PROVIDED
- VHC Vitamin-D Quantitative Vitamin D Test device (Kit Size: 50 Tests/Box, 25 Tests/Box, 10 Tests/Box)
- Sample Buffer (Two Bottles of 6ml in 50 Tests/Box; one Bottle of 6ml in 25 Tests/Box; and 1 Bottle of 3ml in 10 Tests/Box)
- UniSampler blood sample collector 10 µl (50 Devices in 50 Tests/Box; 25 Devices in 25 Tests/Box; and 10 Devices in 10 Tests/Box)
- RFID Card – 1
- Instructions for use – 1
MATERIALS REQUIRED BUT NOT PROVIDED
- Timer or clock
- Lancet
- Alcohol Swab
- VITALITY HEALTH CHECK Health Reader (VHC Reader) – to be purchased separately
STORAGE AND STABILITY
The test device should be stored at 4ºC to 30ºC and will be effective until the expiration date stated on the package. The product is humidity-sensitive and should be used immediately after being open. Any improperly sealed product should be discarded.
PRECAUTIONS
- For in vitro diagnostics use only.
- Do not use the product beyond the expiration date.
- Handle all specimens as potentially infectious.
- Humidity sensitive product, do not open foil pouch until it is ready to be tested.
QUALITY CONTROL
Good Laboratory Practice recommends the daily use of control materials to validate the reliability of device. If control values do not fall within established range, assay results are invalid. Control materials which are not provided with this test kit are commercially available.
The VHC Vitamin-D Quantitative Vitamin D Test provides a built-in process control with a different antigen/antibody reaction at the control region (C). This control line should always appear regardless the presence of Vitamin D. If the control line does not appear, the test device should be discarded and the obtained result is invalid. The presence of this control band in the control region serve as 1) verification that sufficient volume is added, 2) that proper flow is obtained.
CAUTION: VHC Vitamin-D Quantitative Vitamin D Test has been designed for “Decision-Point” Finger-prick Blood (or Serum) samples ONLY. NO Anticoagulated Blood or Plasma samples should be used for testing VHC Vitamin-D Quantitative Vitamin D Test as Anticoagulants will impact the test results.
SPECIMEN COLLECTION AND PREPARATION
- Wash your hand thoroughly and dry completely.
- Rub and Wipe your ring or middle finger of non-dominant hand.
- Using safety lancet puncture the side of your finger.
- Collect 10 µl blood using Blood Collector (see instructions below) and perform testing immediately.
INSTRUCTIONS TO USE UniSampler DEVICE
[table id=4 /]CAUTION:
- Through mixing of blood with Sample Buffer is “EXTREMELY” important to get correct result. This can be determined by checking the uniform red color of pre-mix blood in Collection Tube and Blood Collector.
- Pressing of UniSampler blood sampler should be “GENTLE” to get three full drops of pre-mix blood into the sample well (S).
PROCEDURE
- Bring all materials and specimens to room temperature.
- Remove the test card from the sealed foil pouch and place it on a hard-flat surface.
- Follow Instructions to use UniSampler Device.
- After applying 3 drops of pre-mix blood into the sample well (S), read and record the results at 15 Minutes by VITALITY HEALTH CHECK Health Reader (VHC Reader).
ALTERATIVE SERUM PROTOCOL
VHC Vitamin-D Quantitative Vitamin D Test has been designed for human finger-prick blood. However, Serum sample can be used for testing. Instead of taking finger prick blood with blood collector, apply 5µl of serum into the Collection Tube using Micropipette (not provided with the Kit) and follow “Instructions to Use UniSampler Device”.
Important Note: Result after 15 minutes may not be accurate.
QUANTITATIVE DETECTION USING THE VHC-READER
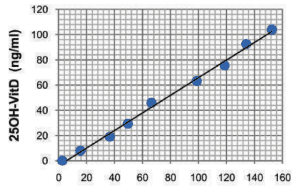
[table id=5 /] STANDARD CURVE USING VITALITY HEALTH CHECK HEALTH READER (VHC-READER)A typical standard curve is illustrated her. The reading AU is automatically converted into ng/ml in VHC Reader.
PERFORMANCE CHARACTERISTICS
Sensitivity
The sensitivity of VHC Vitamin-D Quantitative Vitamin D Test is 3ng/ml (7.5nmol/l). The sensitivity was determined by calculating the mean plus 3.3 times of standard deviation of twenty Vitamin D-free serum tests.
Detection Range
The Detection Range of VHC Vitamin-D Quantitative Vitamin D Test with VHC Reader is from 3ng/ml (7.5nmol/l) to 100ng/ml (250nmol/I)
Accuracy
The accuracy of VHC Vitamin-D Quantitative Vitamin D Test was evaluated using human finger-prick blood samples in comparison with a reference 25OH Vitamin D ELISA assay using corresponding serum samples. The comparison result showed a linear regression with slope of 1.02 and Correlation Coefficient of 92%. In conclusion, VHC Vitamin-D Quantitative Vitamin D Test results of human blood samples showed good agreement with the ELISA results of corresponding serum samples.
The accuracy of VHC Vitamin-D Quantitative Vitamin D Test was also evaluated using 20 serum samples in comparison with LC-MS/MS Assay (“Gold Standard” for 25-OH Vitamin D measurement). The comparison result showed a linear regression with the slope of 0.98 and Correlation Coefficient of 98%. In conclusion, VHC Vitamin-D Quantitative Vitamin D Test results agree closely to the true values generated from LC-MS/MS assay.
Precision
[table id=3 /]
Specificity
30 Vitamin D free serum samples were tested and all showed negative results; suggesting 100% specificity.
No interference and cross reactivity was observed with Bilirubin, Triglycerides, Cholesterol, Vitamin B12 and Vitamin C.
EXPECTED RESULTS
VHC Vitamin-D Quantitative Vitamin D Test is a Rapid Quantitative assay. The test is intended to use for screening individuals to identify Vitamin D level. This assay provides only a preliminary analytical test result. The liquid chromatography with tandem mass spectrometry (LC-MS/MS) assays or quantitative immunoassays are recommended to confirm the analytical result.
REFERENCES
- Holick, MF. Vitamin D statues: Measurement, Interpretation and clinical application. Ann. Epidemiol. 2009, 19(2):73-78.
- Morris HA. Vitamin D: A Hormone for All Seasons – How much is enough? Clin. Biochem. Rev., 2005, 26:21-32.
- Moyad MA. Vitamin D: a rapid review. Dermatol Nurs. 2009, 21:25-30.
- Zerwekh JE. Blood biomarkers of vitamin D status. Am J. Clin Nutr. 2008, 87:1087S-91S.
- Schöttker B, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014, 348:g3656.
- Worm N. Heilkraft D: Wie das Sonnenvitamin vor Herzinfarkt, Krebs und anderen Zivilisationskrankheiten schützt. systemed Verlag, Lünen. 2010, p12.
 Jungbrunnen – Fountain of Youth GmbH
Jungbrunnen – Fountain of Youth GmbH
Cantianstrasse 23
D-10437 Berlin, Germany
info@jungbrunnen.co
www.vitality-health-check.com – www.jungbrunnen.co
Version No: 1/03-04-2017 – Pack Insert No:1 – VHC Vitamin-D Quantitative Vitamin D Test
NOTE: THE ORIGINAL/REFERENCE VERSION OF THIS DOCUMENT IS THE ENGLISH VERSION.